Itay Rousso's Lab
Nanomechanics and dynamics of retrovirus replication
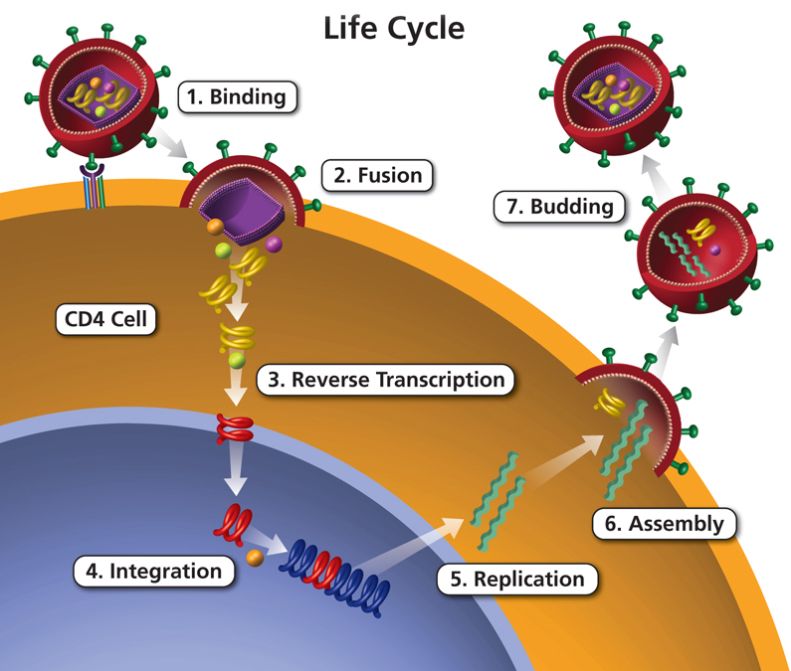
Enveloped retroviruses are complex and efficient self-assembled complexes that are highly evolved to spread infection. In the body, HIV replication occurs via a series of steps that are referred to the virus life cycle. The life cycle can be divided into seven major steps: (1) binding of the virus to the target cell; (2) fusion between the membranes of the virus and the cell; (3) uncoating of the capsid shell and reverse transcription of the viral genome from ss-RNA into ds-DNA; (4) integration of the genome with the target cell genome; (5) assembly of a new virus; (6) budding of the virus; and (7) maturation of the immature virus into a mature and infectious virus. Our research is focused on the physical and mechanical mechanisms underlying the virus replication cycle.
We started by noting that the fusion of viral particles with the membrane of their host cell, as well as their subsequent disassembly inside the cell are likely to depend on their mechanical properties. We postulated that, given that their survival outside the cell poses constraints that are different from those encountered inside the cell, these properties are likely to change at different stages of virus replication. Drawing on these notions, we measured the mechanical properties of immature and mature leukemia viruses (MLV) using AFM. Notably, we found that mature viral particles are twofold softer than their immature counterparts. These results, which represent the first analysis of the mechanical properties of an animal virus, raise the possibility that the overall weakening of the viral structure with its maturation may play a role in cell entry.
Following budding from the cell, retrovirus particles undergo a maturation process that is required for their infectivity. This process is induced by enzymatic cleavage of the Gag polyprotein by a virus encoded protease (PR) into three main structural proteins: matrix (MA), capsid (CA), and nucleocapsid (NC), and is accompanied by massive morphological rearrangements. The other major structural protein of retroviral particles, Env, forms trimeric complexes that mediate membrane fusion. Despite substantial progress in morphological and biochemical characterization of the retroviral lifecycle, many key issues related to virus production, maturation, and entry into cells remain elusive. A virion must satisfy several potentially conflicting demands during its lifetime – spontaneous assembly during budding, durability in the outside environment, and then efficient membrane fusion and subsequent disassembly upon entry into a target cell. Our research addresses some of the issues concerning cell entry and budding of retroviruses by seeking to understand the mechanical principles underlying these processes.
To obtain a deeper insight into the relationship between mechanical properties
and function, we focused on HIV, for which a broad range of research tools exists. Using
AFM to perform indentation assays on virus particles, we found that not only the
mature and immature forms differ in their stiffness, as we observed in MLV, but this
difference is much greater (14-fold). Based on this result, we proposed that the
markedly enhanced stiffness of the shell of immature HIV viruses precludes structural
deformations necessary for efficient fusion with the cell membrane, explaining the poor
ability of immature HIV particles to enter cells. To further test the inverse correlation
between stiffness and cell entry, we measured the stiffness of immature viruses in
which the cytoplasmic (CT) domain (150 aa long) of gp41 was truncated. Such a
truncation was known to significantly increase the ability of immature HIV to enter cells.
We found that truncation of this domain indeed led to a dramatic decrease in the
stiffness of the immature viruses, bringing it close to that of mature HIV particles that
can enter cells with high efficacy. Overall, our studies support a functional connection
between HIV-1 particle stiffness and the control of virus-cell fusion.
can be integrated into the target cell genome. The mechanism that triggers uncoating is
a pivotal question of long standing. In a pioneering studies, we have characterized the
effect of stabilizing mutations, the capsid-targeting inhibitor PF74, and the capsidbinding host protein cyclophilin A on the mechanical stability of the HIV-1 capsid. Using
self-assembled capsid protein structures allowed us to measure capsid stiffness in
isolation from its contents, which revealed that the empty WT capsid is extremely soft
and that its core contents have a reinforcing effect on it. This finding of an extremely
soft capsid fits well with uncoating models in which DNA synthesis mechanically
destabilizes the capsid up to a breaking point. As our results show, capsid-stabilizing
mutations significantly stiffen the capsid structure, which results in decreased infectivity
in several of the mutants. We then characterized the mechanical properties and
morphology of isolated cores during reverse transcription. We showed that the pressure
inside the capsid increases during an early stage of reverse transcription. Subsequently,
the stiffness of the capsid drops precipitously to a value below that of a pre-reverse
transcription core, and the capsid undergoes partial or complete rupture near the
narrow end of the conical structure. The application of AFM technologies to study
purified HIV-1 cores represents a new experimental platform for studying capsid
disassembly and HIV-1 uncoating. This platform will be further deployed in the current
research application784.
The assembly and budding of new virus particles are fundamental steps in the retrovirus replication cycle. For HIV and MLV, both of these processes occur at the plasma membrane of the infected cell. Retroviral budding has been extensively studied using EM methods. Although these studies provide detailed structural information, they are incapable of yielding dynamic information. Dynamic information can be extracted from biochemical assays but is limited by ensemble averaging. Thus, despite the substantial progress made in the characterization of retroviral budding, the underlying physical mechanism is not fully understood. In particular, the mechanism by which the virus overcomes the energy barrier associated with the formation of high membrane curvature during viral budding is unknown.
To acquire high-resolution structural information in real time and at the singleparticle level, we employed time-lapse atomic force microscopy to follow the budding of individual MLV viruses from NIH/3T3 cells. We found that MLV bud formation follows at least two kinetically distinct pathways, with the majority of the virions being assembled and exiting from the cell in a slow process (>45 minutes) and the remaining population exhibiting a much faster kinetics (<25 minutes). Intriguingly, some of the viral particles appeared to be moving in a specific direction along cellular stress fibers. This observation suggested that virus particles might interact with the cytoskeleton of the infected cell during assembly and budding.
To follow this latter observation, we used torsion-mode AFM (which improves the contrast of the cytoskeletal features underneath the cell membrane) to image the cell cytoskeleton during viral budding. We found that the assembled virions were often connected to highly dynamic star shaped filaments, presumably of actin, which varied in size over the duration of budding. These filamentous structures assembled simultaneously or immediately after the beginning of budding, and disappeared as soon as the nascent virus was released from the cell membrane. Analysis of sections of cryopreserved infected cells by transmission electron microscopy revealed similar structures emerging from every nascent virus. Finally, employing immuno-fluorescence microscopy we were able to confirm the molecular identity of these filaments as actin.
To address the question of what functional role this apparent remodeling of actin plays in retroviral budding, we compared the assembly and budding of virus-like particles (VLPs) made of wild-type Gag to that of VLPs made of Gag chimeras in which the nucleocapsid domain that is thought to bind actin was replaced by a leucine-zipper domain. Cytoskeleton remodeling was not observed during formation of the leucinezipper VLPs. Notably, budding of particles carrying the modified nucleocapsid domains was an order of magnitude slower than that of those carrying the wild-type domain. We therefore conclude that retroviruses take advantage of this mechanism to expedite their assembly and budding.
Currently, we are studying the role of the ESCRT machinery in HIV-1 budding, using ultra-fast AFM system, which enables us to obtain AFM images of infected live cells at 4-6 seconds temporal resolution.