Stromal cell-induced immune regulation in a transplantable lymphoid-like cell constructs
In collaboration with Prof. Cohen Smadar, The Department of Biotechnology Engineering, Ben-Gurion University of the Negev, Beer-Sheva, Israel
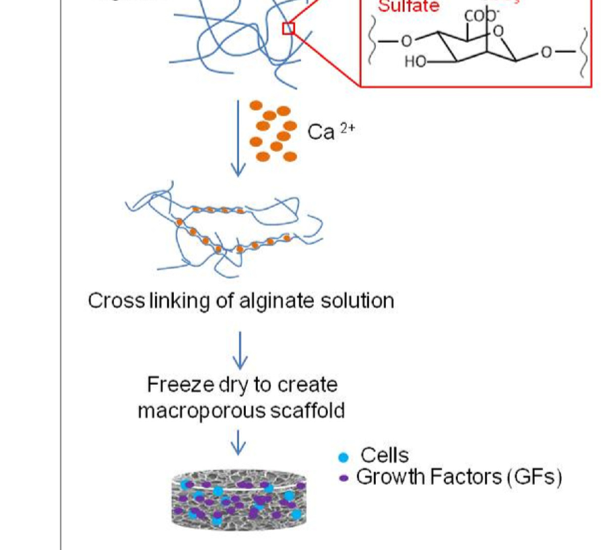
Engineering of cell-based constructs for treating a variety of immune-related diseases by local transplantation of the cells in a pre-designed matrix is an emerging therapeutic approach, which can potentially reduce the side effects associated with systemic cell injection. Stromal cells have been shown to exert immunosuppressive properties and thus can be exploited for autoimmune regulation and cell transplantation. We demonstrated a stromal cell-based construct, which serves as a lymphoid-like organ with immune regulatory characteristics whereby stromal cells are co-seeded with dendritic cells (DC) in a macro-porous alginate scaffold containing the encephalitogenic myelin-derived peptide, proteolipid protein (PLP). Alginate scaffolds herein are replacing the extracellular matrix (ECM), providing both a physical support and biological cues for the seeded cells and closing the gap to in vivo conditions (Kaminer et al., 2010). In a recent study by Orr et al., 2016, we investigated the feasibility of generating an immunoregulatory environment in a highly vascularized macroporous alginate scaffold by affinity-binding of the transforming growth factor-β (TGF-β) in a manner mimicking its binding to heparan sulfate. Using this device to transplant allofibroblasts under the kidney capsule resulted in the induction of local and peripheral TGF-β-dependent immunotolerance, characterized by higher frequency of immature dendritic cells and regulatory T cells within the device and by markedly reduced allofibroblast-specific T-cell response in the spleen, thereby increasing the viability of the transplanted cells. We thus demonstrate a novel platform for transplantation devices, designed to promote an immunoregulatory microenvironment suitable for cell transplantation and autoimmune regulation. Current studies are performed to determine the capacity of immunoregulatory 3D scaffolds to protect from the rejection of allogeneic insulin-producing cells in animal models of diabetes.