Immune mechanisms in aging and neurodegenerative diseases.
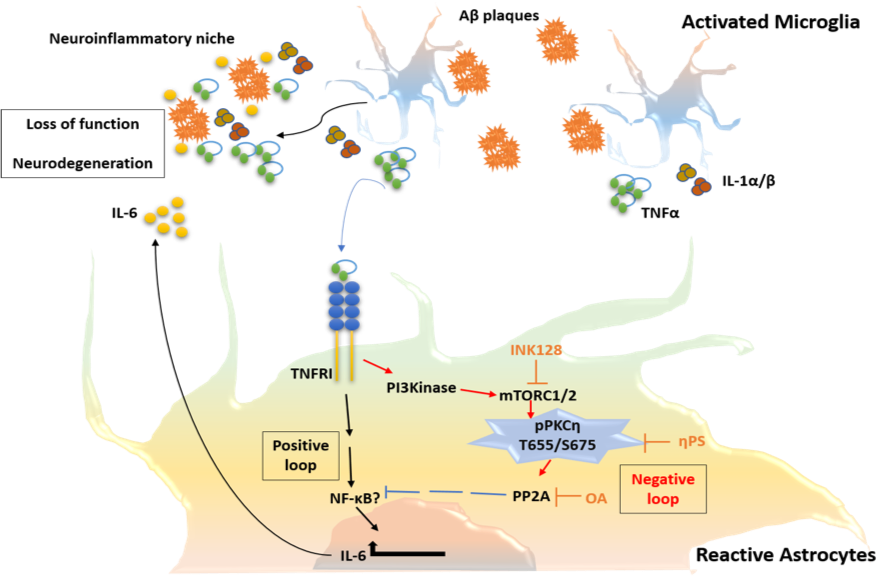
Alzheimer’s disease (AD) is the most common form of dementia, with prevalence progressively increasing with aging. Pathological hallmarks of the disease include accumulation of amyloid-beta (Aβ) peptides and neurofibrillary tangles in the brain associated with glial activation and synpatotoxicity. In addition, AD involves peripheral and brain-endogenous inflammatory processes that appear to enhance disease progression.
Our ongoing studies, using animal models of Alzheimer’s disease, unveil the inflammatory role of microglial subsets, particularly those holding a dialogue with peripheral leukocytes, in the disease process.
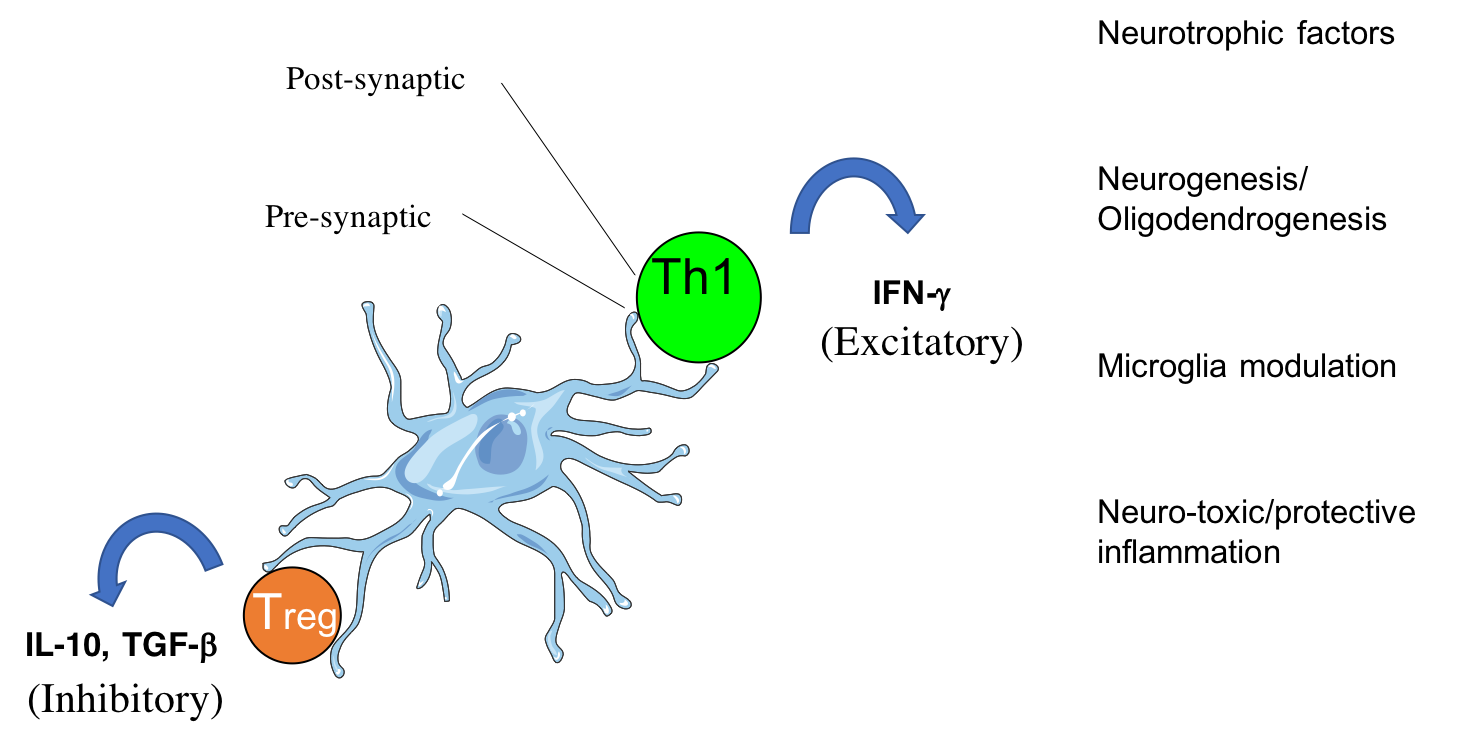
CD4 T cells form cytotoxic or beneficial effector functions within the CNS (Mittal et al., 2019; Eremenko et al., 2019)
In addition, we study how the activation of astrocytes, being key modulators of the blood-brain-barrier and the neuronal network, impacts the disease process.
Apart from activation of an innate immune response in the brain, more than a decade ago a new therapeutic paradigm has emerged for AD, namely the activation of the adaptive immune system directly against the self-peptide Aβ, aiming at lowering its accumulation and neurotoxicity. This was the first time that a brain peptide was used to vaccinate human subjects in a manner similar to classic viral or bacterial vaccines. The vaccination approach took several turns, from initially active to passive and then back to modified active vaccines. As the two first approaches to date failed to show sufficient efficacy, the latter is presently being evaluated in ongoing clinical trials. In the review (CD4 T cells in immunity and immunotherapy of Alzheimer’s disease. Immunology. 2013), we summarize the immunogenic characteristics of Aβ in human and mice and discuss past, present and future Ai-based immunotherapeutic approaches for AD. We emphasize potential pathogenic and beneficial roles of CD4 T cells in light of the general decline in T-cell responsiveness evident in aging. More recently, the studies by Fisher et al., 2014, Strominger et al., 2018, Mittal et al., 2019 and Eremenko et al., 2019, provided mechanistic insights to the migration of CD4 T cells into the choroid plexus and into the brain parenchyma and highlight implications to brain immunity and repair. Current work is devoted to further understanding the dialogue between the immune system and the brain and to generate immunotherapeutic T cells as targeted drug-delivery vehicles.
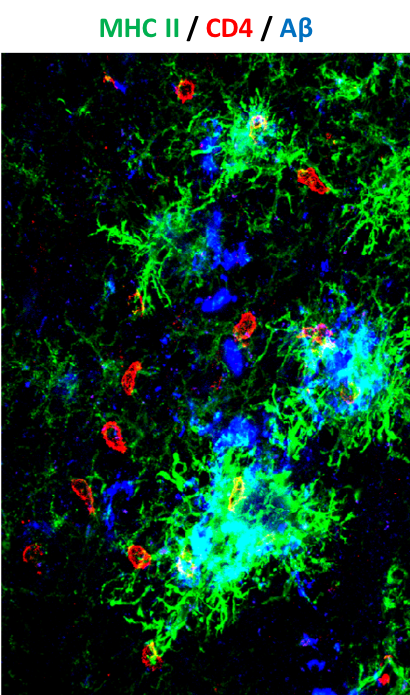
Activated leukocytes (red) attack amyloid plaques in the brain of a mouse model of Alzheimer disease
(Strominger et al., 2018)
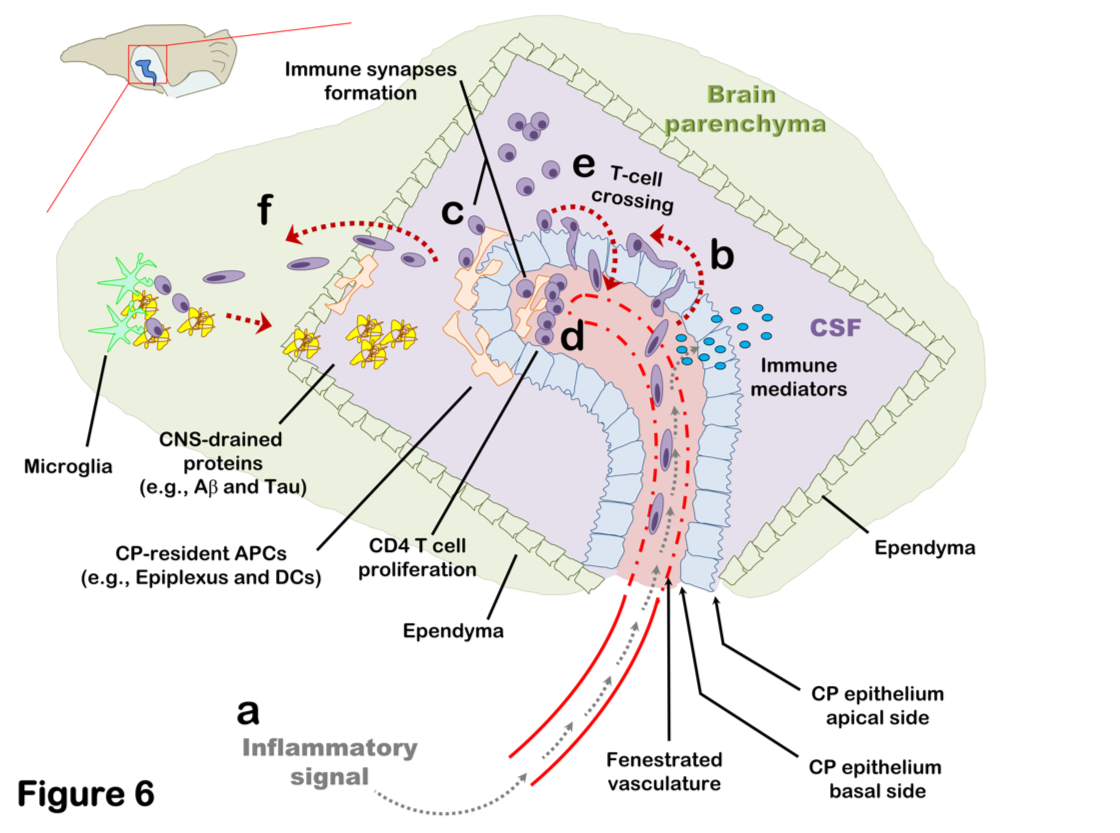
Brain-Immune interactions at the choroid plexus: A semi-lymphoid structure that regulates brain inflammation
(Strominger et al., 2018; Mittal et al., 2019; Eremenko et al., 2019)
Immune biomarkers of aging and age-related diseases
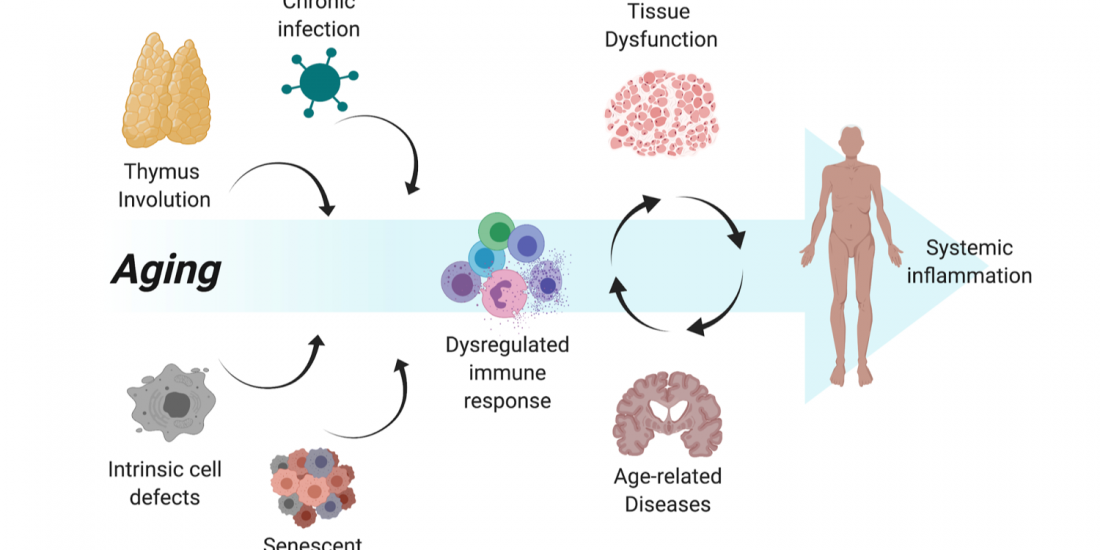
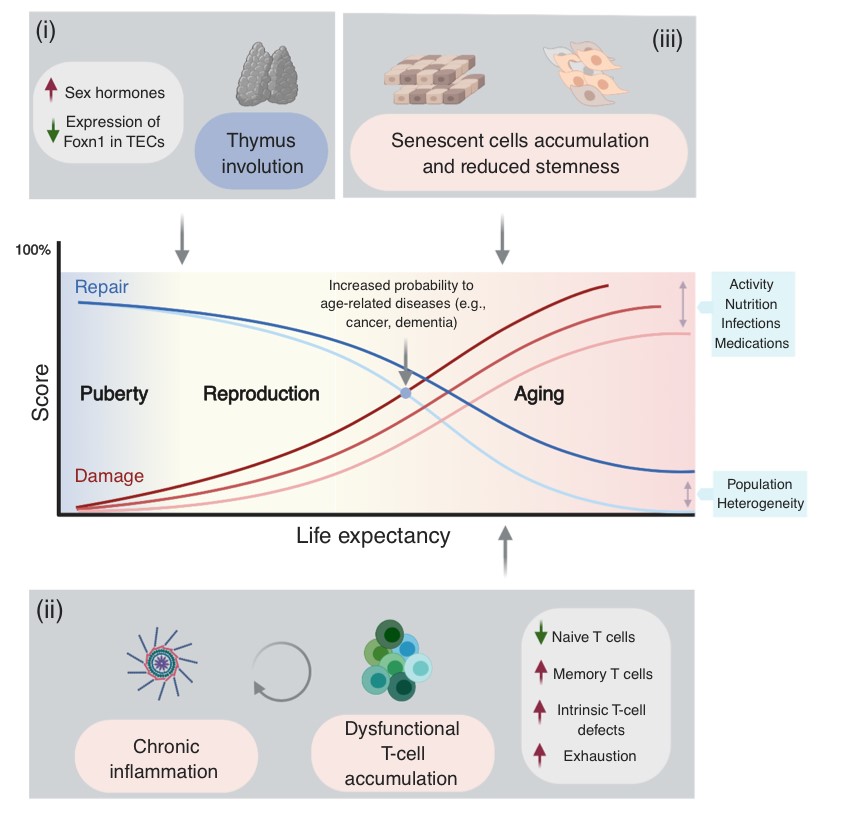
Among the many possible aging-related factors involved in the development of age-related diseases, senescence of the immune system may significantly enhance inflammation and neuronal damage in the brain and thus facilitate cognitive decline. A direct consequence of immunosenescence is the increased frequency of systemic infections and, in fact, many who die with AD have previously suffered from a severe infection and/or chronic inflammation.
While chronic brain inflammation progresses throughout the disease, the specific arm of the immune system, i.e., brain-specific lymphocytes, may also be stimulated. In contrast to previous assumptions, our findings in people with AD demonstrated that a specific immune response to A-beta is indeed significantly induced in both elderly individuals and patients with AD as compared to middle-aged individuals (Monsonego et al., 2003). The nature and role of this immune response to A-beta is yet to be investigated, and may result in new diagnostic and immunotherapeutic approaches. More recently we have shown that aging results in the accumulation of effector CD4 T cells exhibiting extremely dysregulated functions (Harpaz et al., 2017). Since these dysregulated T cells may significantly impact inflammatory processes in the brain of people with AD, in collaboration with Prof. Esti Yeger-Lotem and Prof. Nir Friedman we initiated a single-cell RNA analysis of leukocytes to reveal how aging impacts the landscape of lymphocytes as a key process that may underlie declined immunity and chronic inflammation in the elderly (Elyahu et al., 2019; Elayhu et al., 2020). Current research aims to further reveal the cellular and molecular characteristics of dysregulated CD4 T cells in aging and neurodegenerative processes both in human and in animal models.
Immune cell residence and function within the central nervous system
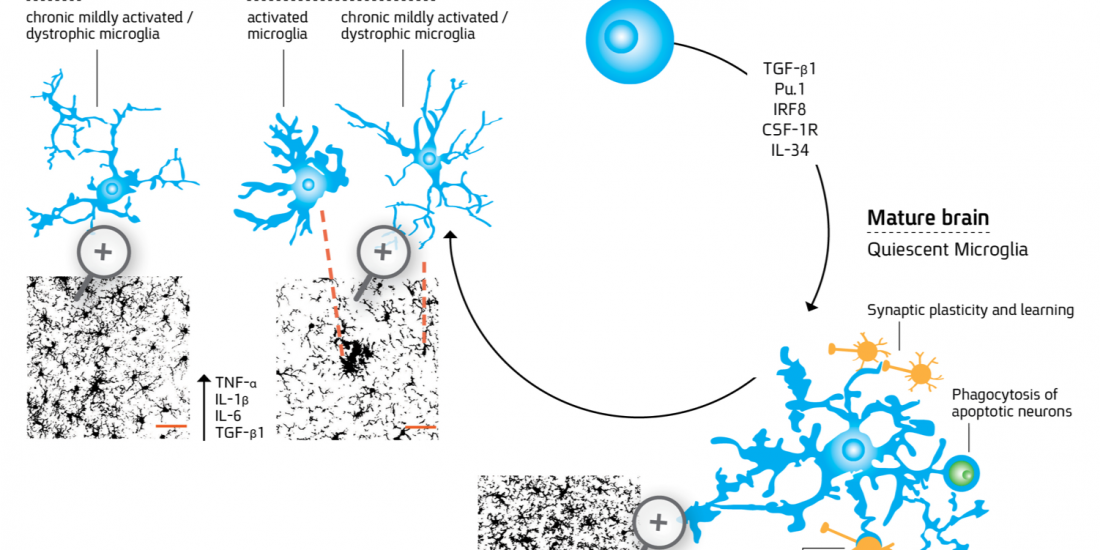
Microglia are myeloid-derived cells that colonize the central nervous system (CNS) already at early stages of development and constitute up to 20% of the glial populations throughout life. While extensive progress has been recently made in identifying the cellular origin of microglia, the mechanism whereby the cells acquire the unique ramified and quiescent phenotype within the CNS milieu and the changes the cells undergo in aging and through the course of CNS disorders requires further research. For more details about microglial differentiation and their role in disease see the following references: Abutbul et al., 2012, Baron et al., 2014; Andreasson KI et al., 2016.
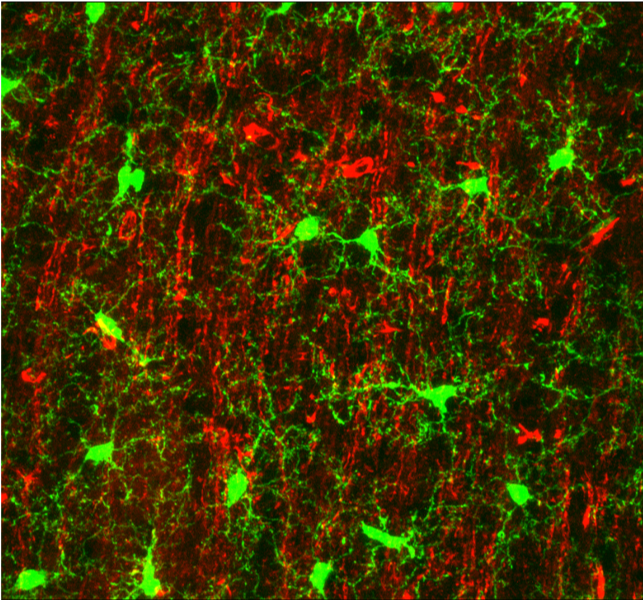
Microglia processes (green) form contacts with neurons (red) in the healthy brain
(Baron et al., 2014)
Our current studies are targeted to reveal the differentiation of microglia at early stages of embryogenesis using genetically-modified zebrafish (in collaboration with Dr. Niva Russek-Blum, The Dead Sea and Arava Science center). In addition, we study the functional properties of the cells in animal models of neurodegenerative diseases (for more details see “Immune mechanisms in aging and neurodegenerative diseases”).
Stromal cell-induced immune regulation in a transplantable lymphoid-like cell constructs
In collaboration with Prof. Cohen Smadar, The Department of Biotechnology Engineering, Ben-Gurion University of the Negev, Beer-Sheva, Israel
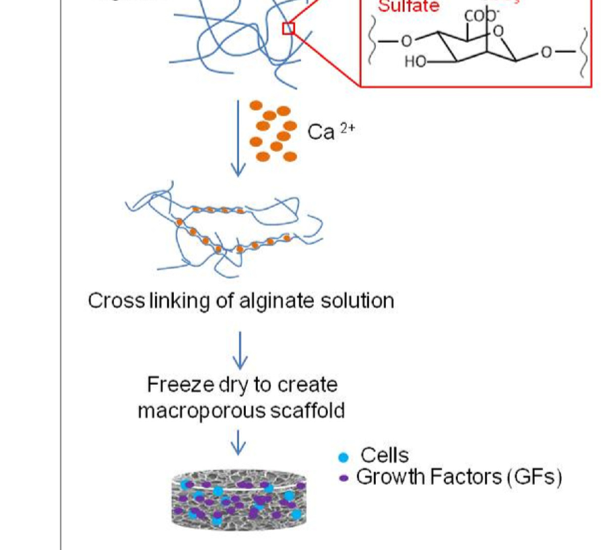
Engineering of cell-based constructs for treating a variety of immune-related diseases by local transplantation of the cells in a pre-designed matrix is an emerging therapeutic approach, which can potentially reduce the side effects associated with systemic cell injection. Stromal cells have been shown to exert immunosuppressive properties and thus can be exploited for autoimmune regulation and cell transplantation. We demonstrated a stromal cell-based construct, which serves as a lymphoid-like organ with immune regulatory characteristics whereby stromal cells are co-seeded with dendritic cells (DC) in a macro-porous alginate scaffold containing the encephalitogenic myelin-derived peptide, proteolipid protein (PLP). Alginate scaffolds herein are replacing the extracellular matrix (ECM), providing both a physical support and biological cues for the seeded cells and closing the gap to in vivo conditions (Kaminer et al., 2010). In a recent study by Orr et al., 2016, we investigated the feasibility of generating an immunoregulatory environment in a highly vascularized macroporous alginate scaffold by affinity-binding of the transforming growth factor-β (TGF-β) in a manner mimicking its binding to heparan sulfate. Using this device to transplant allofibroblasts under the kidney capsule resulted in the induction of local and peripheral TGF-β-dependent immunotolerance, characterized by higher frequency of immature dendritic cells and regulatory T cells within the device and by markedly reduced allofibroblast-specific T-cell response in the spleen, thereby increasing the viability of the transplanted cells. We thus demonstrate a novel platform for transplantation devices, designed to promote an immunoregulatory microenvironment suitable for cell transplantation and autoimmune regulation. Current studies are performed to determine the capacity of immunoregulatory 3D scaffolds to protect from the rejection of allogeneic insulin-producing cells in animal models of diabetes.
Mechanisms of stress-induced autoimmunity and neuroinflammation
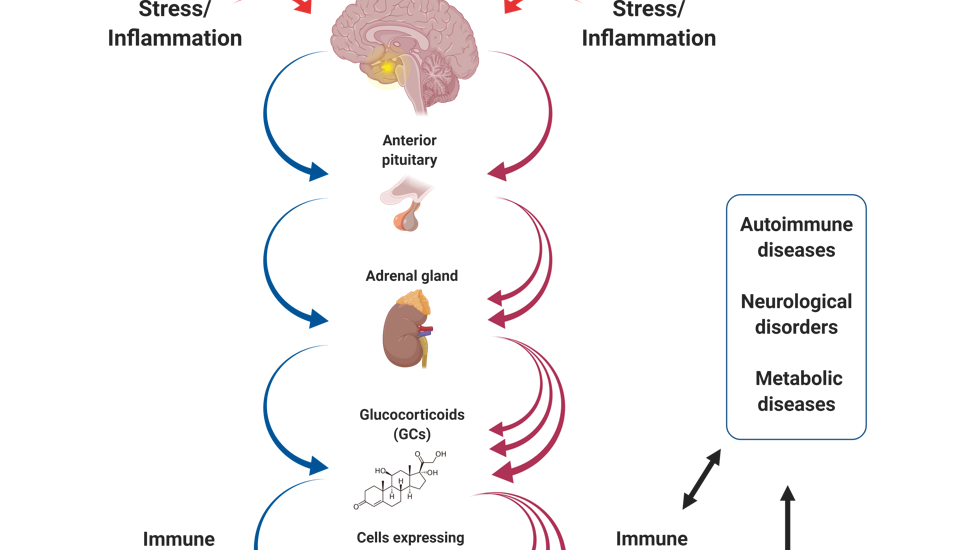
It has been well established that stress may substantially affect the homeostatic regulation of the immune system. In most animal models studied thus far, stressful triggers such as fear, maternal deprivation, social threat or physiological challenge have been shown to induce immunosuppression associated with increased susceptibility to allergies and infectious diseases. These effects are mediated by the hypothalamic-pituitary-adrenal (HPA) axis, a complex network linking the nervous, endocrine and immune systems.
In line with the critical role of the HPA axis in suppressing pathogenic autoimmunity and clinical observations linking stress with disease exacerbation, our recent study (Harpaz et al., 2013) shows that prolonged stress exposure worsens, rather than ameliorates, clinical symptoms in a mouse model of multiple sclerosis; a phenomenon, however, which could be prevented by blocking glucocorticoid signaling throughout the stress exposure period. We also show that glucocorticoid levels under basal conditions are significantly lower in male than in female mice, associated with exacerbated disease symptoms. Finally, we show that stress decreases the Treg/Teff ratio, and increases the Th1-Th17/Th2 ratio, within the Teff-cell subsets. Taken together, our findings raise the possibility that while the HPA axis provides immunosuppression under basal conditions (i.e., in non-stressed females), prolonged exposure to chronic stress results in an attenuated CORT response to stimuli primarily in pro-inflammatory effector CD4 T cells, predisposing to higher susceptibility to pathogenic autoimmunity.
The notion that overactivation of the HPA axis disrupts a key homeostatic regulation of inflammation, prompt us to broaden our research goals. Current studies in the lab thus address the impact of chronic stress on gut inflammation, autoimmunity, brain inflammation and psychiatric disorders.